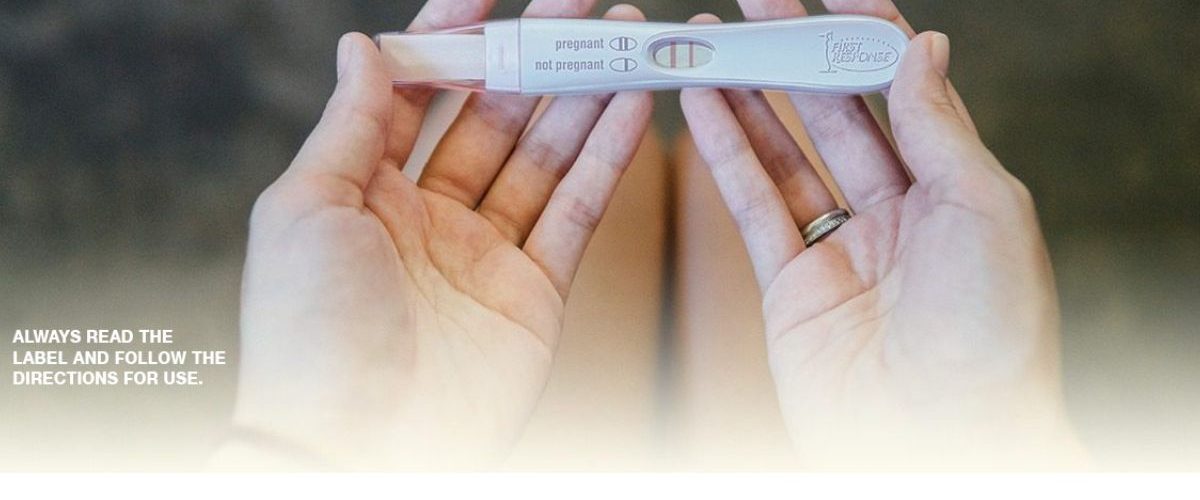
Short of visiting a doctor, a pregnancy test is the best way to answer the question “Am I pregnant?”. Learn how FIRST RESPONSE™ pregnancy tests detect the hCG hormone levels in a woman’s urine to determine if she is pregnant and understand how early detection is better for you and better for your baby.
DETECTING PREGNANCY HORMONE
From the earliest moments of conception, your body is already starting to undergo monumental changes. One of the very first is the production of the pregnancy hormone, hCG (human Chorionic Gonadotropin), which can be detected in your blood and in your urine. The amount of hCG in your body increases rapidly. It doubles every 36 to 48 hours as your pregnancy progresses, reaching its peak at eight to ten weeks. Pregnancy tests work by detecting hCG levels in your urine. When a woman’s urine comes in contact with the specially treated strip on a pregnancy test stick, results appear within minutes, indicating whether or not hCG, the pregnancy hormone, has been detected.
ABOUT PREGNANCY TESTS
Some at-home pregnancy tests are unable to detect hCG in urine sooner than the first day of a missed period. As Australia’s leading at-home pregnancy test brand, advancements in sensitive technology have enabled FIRST RESPONSE™ Early Result In-Stream Pregnancy Test and FIRST RESPONSE™ Digital Pregnancy Test to detect the level of HCG in your body 6 days before a missed period1,2.
1In-Stream Pregnancy Test Kit: The following claim “In Clinical testing, First Response detected hormone levels in 62% of women, 6 days before the missed period (5 days before the day of expected period), in 78% of women 5 days before their missed period, in 87% of women 4 days before their missed period, in 98% of women 3 days before their missed period and in 99% of women 2 days before their missed period” is based on the unpublished study ‘Determination of incidence of positive human chorionic gonadotropin signal in urine samples from non-pregnant women of pre-, peri- and post-menopausal age’ sponsored by Church & Dwight Co. Inc., and reported by Kelly Zhang, William Hooper & Annahita Ghassemi.
2Digital Pregnancy Test Kit: The following claim “ In Clinical testing, First Response detected the pregnancy hormone levels in 60% of pregnant women, 6 days before the missed period (5 days before the day of expected period), in 86% of pregnant women 5 days before their missed period, in 96% of pregnant women 4 days before their missed period, >99% of pregnant women 3 days before their missed period” is based on the unpublished study ‘Detection of hCG in Early Pregnancy Conceptive Cycles’ sponsored by Church & Dwight Co. Inc., and reported by Timothy Snowden.
3Ovulation Prediction Strips (LH): The following claim “Over 99% accurate in detecting LH surge in Laboratory Testing” is based on the unpublished ‘Clinical Evidence Report’ by Kyung-ah Kim, sponsored by Princeton Biomeditech Corporation.
4Pregnancy Detection Strips (hCG): The following claim “Over 99% accurate in detecting hCG surge in Laboratory Testing” is based on the unpublished ‘Assay Precision and Accuracy’ study by Kyung-ah Kim, sponsored by Princeton Biomeditech Corporation.
5Ovulation Prediction Sticks (LH) The following claim “Ovulation test is over 99% accurate in detecting LH surge in Laboratory Studies” is based on the unpublished study ‘First Response/ Answer Ovulation Predictor Test-Analytical Accuracy Evaluation’ sponsored by Church & Dwight Co. Inc., and reported by Mary Beth Boyle.
6First Response Lubricant
6aClinically shown to be fertility friendly or spermicide free’ claim is based on the studies shown in the article ‘Sperm toxicity of nonspermicidal lubricant and ultrasound gels used in reproductive medicine’ published on Fertility & Sterility Vol.95 No.2, February 2011; 835–6. 2011 by American Society for Reproductive Medicine, Published by Elsevier Inc.
6bIsotonic, mimics your fertile fluid’ claim is based on the studies shown in the article ‘Effect of vaginal lubricants on sperm motility and chromatin integrity: a prospective comparative study’ published on Fertility & Sterility Vol.89 No.2, February 2008; 375-9 2008, by American Society for Reproductive Medicine, Published by Elsevier Inc.